1,3-dipolar cycloaddition reactions have become recognized for their ability to provide efficient access to pharmaceutically relevant heterocyclic products. A challenge in this area is that 1,3-dipoles are often reactive substrates, and, if highly substituted, can themselves be the product of an involved, multistep synthesis. To address this issue, we have designed new routes to generate 1,3-dipoles directly from available substrates. For example, we have developed a method to synthesize 1,3-oxazolium-5-oxides (Münchnones), via the palladium catalyzed coupling of simple imines, acid chlorides and CO. Alternatively, the one-pot reaction of imines, acid chlorides, and phosphonites has been found to generate a new class of phosphorus-based 1,3-dipolar cycloaddition reagent. These isomeric forms of Wittig reagents, undergo cycloaddition reactions in an analogous fashion to Münchnones. However, these 1,3-dipoles (Phospha-Münchnones) can be generated in one pot, in a modular fashion, and directly from available reagents, suggesting their potential general utility in heterocycle synthesis.
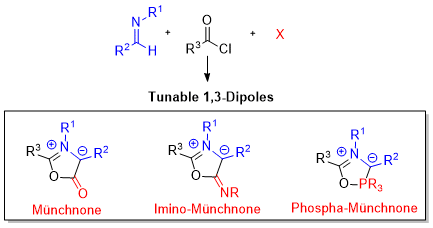
By coupling the one step formation of these dipoles with their cycloaddition, these platforms can provide modular methods to generate a range of heterocyclic products. We are currently using and studying the reactivity of these phospha-Münchnones in regioselective and enantioselective cycloaddition reactions.